Laboratory of Molecular Bioenergetics
Center For Genetic Diseases
The Chicago Medical School
| Publications | Research | Laboratory Members | Method Links |
 |
Laboratory of Molecular Bioenergetics Center For Genetic Diseases The Chicago Medical School | |||
|
|
|
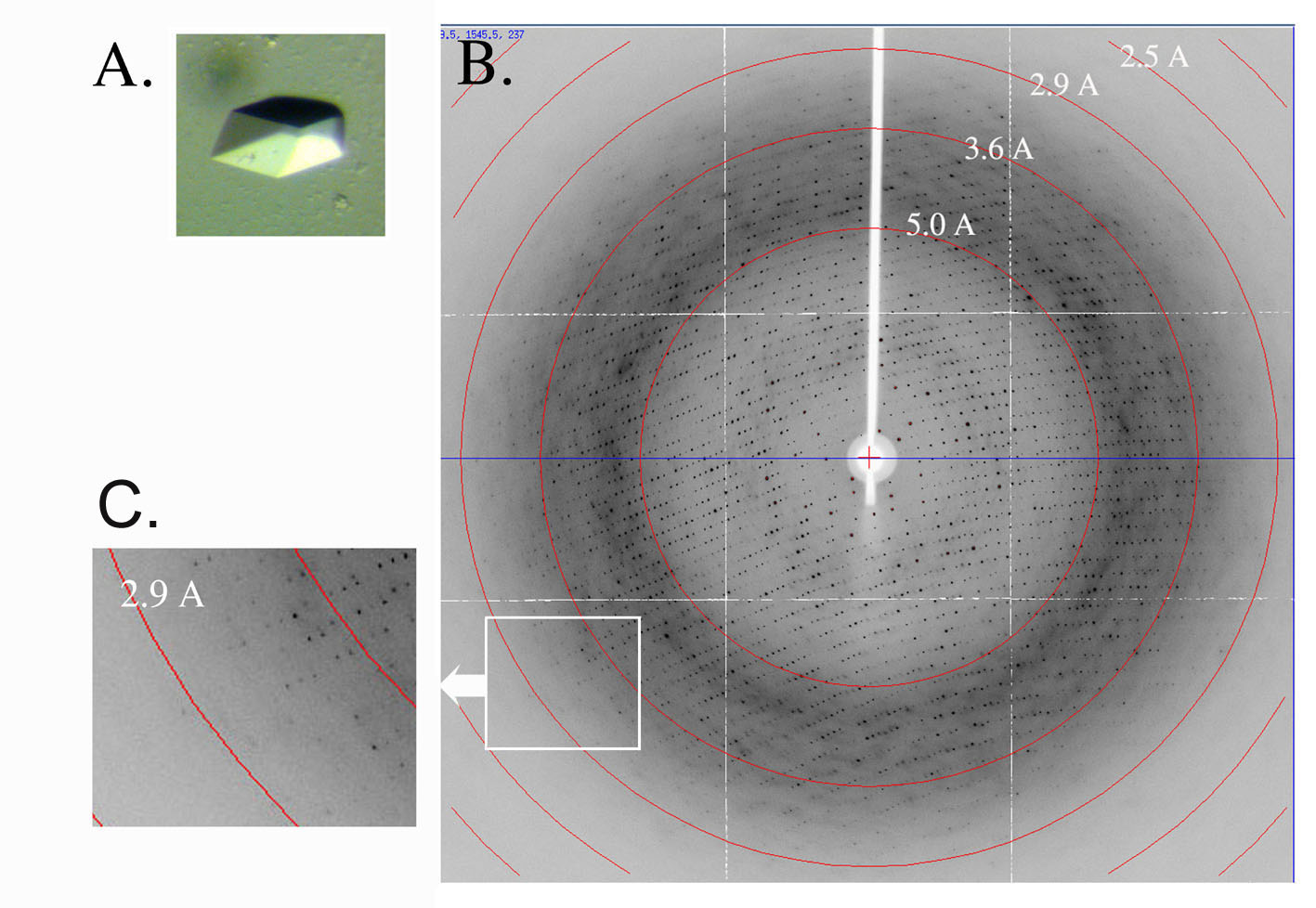
We were not alive in the 15th century during the Renaissance - when great artists such as Donatello, da Vinci, and Michelangelo walked the earth. Nor did we experience the times of the great inventors, such as Edison and the Wright brothers. But we are very fortunate to live in the Golden Age of Molecular Biology. In a short 50 years, we have seen the structure of DNA, to the molecular structures of proteins, to the complete nucleotide sequence of the human genome. This is indeed a very exciting time for the observer, but even more so for the participant. Teaching: My primary teaching roles for the medical school have been in the areas of DNA and RNA structure, DNA recombination and repair, gene regulation, RNA processing, modification and expression. I also have participated in teaching Medical Biochemistry where I discuss thermodynamics, bioenergetics, mitochondrial diseases, and mixed function oxidases. These areas are all under intense investigation in the laboratory and every effort is made to discuss the current knowledge on the topics. This sometimes meant that information was discussed that is not covered in text books. I currently teach amino acid metabolism and the urea cycle in Medical Biochemistry to both the medical and graduate students. My largest teaching block is in Clinical Genetic. This course includes topics such as risk analysis, chromosomal abnormalities, and ethics. I was the course director, but now only teach in a core graduate school course. This is a broad-based course and I cover topics on protein folding and chaperonins. Research: I have two primary areas of research. The first, and longest running, is in the understanding of the structure, function, and regulation of the mitochondrial ATP synthase. The studies are undertaken using a variety of techniques in the area of yeast genetics, biochemistry, molecular biology, and x-ray crystallography. The x-ray diffraction data is obtained at the Advanced Photon Source in Argonne National Laboratories just outside Chicago. The x-ray crystallography were collaborative studies with Dr. John Walker at the Mitochondrial Biology Unit and Dr. Andrew Leslie at the Laboratory of Molecular Biology, both in Cambridge U.K. and part of the Medical Research Council (MRC). We have recently moved to cryo-electron microscopy in colloboration with Dr. Maofu Liao of Harvard Medical School. The second area of research is in the study of the Juvenile for of Batten Disease. We are engaged in understanding the structure and function of the gene product defected in the juvenile form of Batten disease by a combination of genetic, biochemical and biophysical methods. We are engaged in obtaining the high resolution structure of the gene product defective in this disease, Cln3p.
Please visit the research page for more information on the projects and the photo gallery for images resulting from our work.
Movie: Electron Density map of oligomycin bound to yeast ATP synthase c-ring. The denisty map is colored using the key below.
html5 video converter by EasyHtml5Video.com v4.0 Cryo-Image and Power Spectrum of Yeast ATP Synthase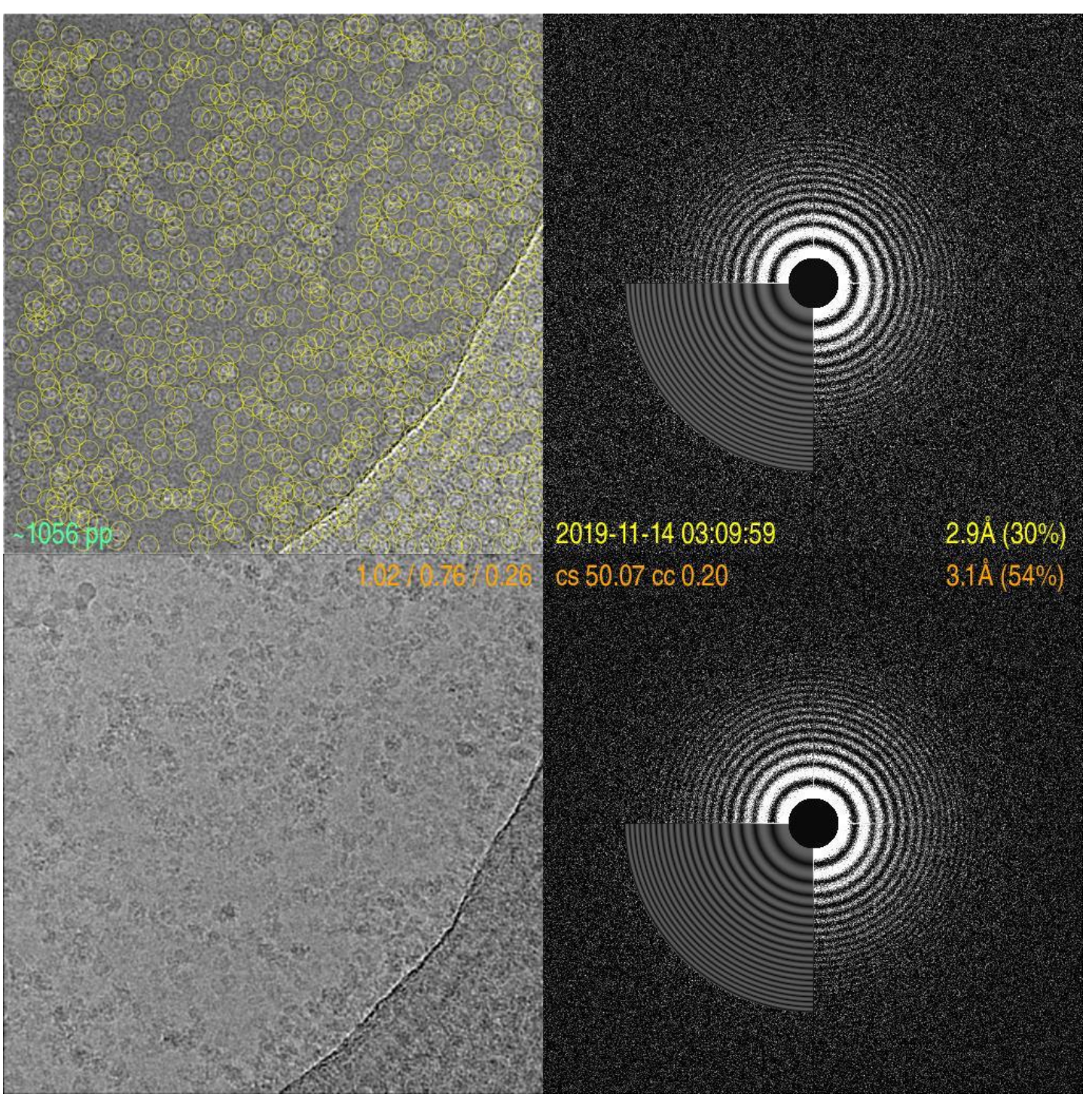
|
NEWS
Current funding from NIGMS - R35GM131731. In Collaboration with the labs of Drs. Maofu Liao's and Dr. Jose Faraldo-Gomez, the manuscript "Bedaquiline inhibits the yeast and human mitochondrial ATP synthases" in Communication Biology details the study that demonstrates with biochemical, molecular dynamic simulation and cryo-EM methods, that bedaquiline binds and inhibits the mitochondrial ATP synthase. This study provides a rationale to design a safer derivative of the antibiotic, Sirturo, used to treat TB. In Collaboration with Dr. Mohamad A. Mikati (Corresponding author, Duke), Dr. Raphael Shiffman (Baylor), Dr. Kristen Herman (Columbia), and Dr. Vijay Pagadala (GlycanTherapeutics, LLC) a study on disease mutations in the sodium/potasium ATPase. The manuscript is entitled "D-DEMO, a distinct phenotype caused by ATP1A3 mutations" In Collaboration with Dr. Anurag Srivastava, the manuscript entitled "A candidate multi-epitope vaccine against SARS-CoV-2" In collaboration with Dr. Maofu Liao, we solved the structure of the ATP synthase embedded in lipid nanodiscs. Science 2018. Dr. Francis Collins - NIH Director - Blogs on the FDA approval of Sirturo. Link FDA grants approval for 1st TB drug in 40 years. Johnson&Johnson was granted approval for Sirturo, aka TMC207, for the treatment in adults with pulmonary multi-drug resistant tuberculosis. see link TMC207 binds to Fo of the ATP synthase in the corresponding oligomycin binding site of the mitochondrial ATP synthase. 10,000 downloads in 3 months of the publication!! Oligomycin frames a common drug-binding site in the ATP synthase. Proc. Natl. Acad. Sci. USA USA 28: 13961-13965, 2012. Get your's at this link. Recognition from Argonne National Labs and GM/CA! Link Welcome two new additions to the laboratory. Dr. Ting Xu is an accomplished crystallographer with 16 pdbs under his belt with studies on proteins from Dengue virus and M. tuberculosis. Ting obtained his Ph.D at Nanyang Technological University in Singapore and has done post-doctoral research at the NIH. We are very excited that Ting will be joining the group. Manjit Maskey obtained his B.S. at St. Cloud University in Minnesota. Manjit then got a M.S. from Iowa State University in the lab of Dr. Richard Honzatko. Manjit has completed some very studies on the binding of hexokinase to the outer mitochondrial membrane. We are excited that Manjit will be joining the lab. Welcome Ting and Manjit! NIH Award: We are very fortunate to have received a 4 year renewal for the NIH project "Structure and Function of the ATP synthase"! Thanks to all involved. Cheers Welcome Michal Bonar to the lab! Michal has a B.S. from U of Illinois, Urbana and an M.S. from McGill in Montreal. PNAS - structure of the oligomycin binding site at 1.9A! LINK Oligomycin Frames a Common Drug Binding Site in the ATP synthase (2012) J. Symersky, D. Osowski, D.E. Walters, and D.M. Mueller, PNAS, in press. Congratulations to all! The structure of the yeast c10-ring was highlighted in Biopolymers - "An Open and Shut Case". Congratulations to Henry Symersky, Vijay Pagadala, Daniel Osowski, Alexander Krah, Thomas Meier, and Jose Faraldo-Gomez - an international effort - for the NSMB paper accepted on 20-March-2012!! "Structure of the c10 ring of the yeast mitochondrial ATP synthase in the open conformation" pdb entries 3U2F, 3U32, 3U2Y, and 3UD0. These are available from the database with the structure factors to be released with the publication of the paper. Thanks to GC/CA-CAT for their continued support and LS-CAT at the APS for their recent support. Congratulations Vijay on your very nice paper describing the purification and properties of the yeast ATP synthase with the GFP-fusion construct. pdf Congratulations to Kalpit on his new journey as a Ph.D. student at RFUMS! We have entered into a collaboration with Thomas Meier and Jose Faralso-Gomez, Max Planck Institute of Biophysics, Frankfurt, Germany, to study Fo of the ATP synthase. More on this later!
Long overdue welcome to Daniel Osowski. Daniel received his B.S. from Illinois State University and currently working on the ATP synthase project. Long overdue welcome to Kristy Shanahan into the lab in the graduate program. Farwell to Vijay and Diana! Vijay completed his Ph.D. and is off to NIH in the research triangle in North Carolina. Diana is off to the Centre de Biochemie Structurale (CNRS) in Montpellier, France, and is engaged to be married this winter. Congratulations! Structure of four uncoupling mutations in F1 ATPase is in JBC. This was truly a multi-person effort. Every author was critical in the completion of this project. Congratulations to all! I, and a reviewer of the manuscript, recommend the movies in the supplement - they help in the visualization of the effects of the mutations. pdf Yamin, congratulations on completing the degree of Ph.D.! Yamin is now working at the University of Chicago as a post-doctoral fellow. (Co-incidentally, former student Hong Shen is also at the U of C.) Yamin has published a very nice study that helps explain the structure/function relationship of the mitochondrial genome integrity mutations. See JBC. Refinement is Complete!! The refinement of the structure of the yeast F1-ATPase in the absence of nucleotides is complete. pdf Vijae was an Awarded American Heart Predoctoral Fellowship for 2006-2007! Collaboration with Dr. Richard Berry in Oxford, U.K. A collaboration has been started with Dr. Berry at Oxford. Dr. Berry performs single molecule studies followed by fluorescence and atomic force microscopy. The studies are a 3 group collaborative study which include Dr. John Walker at the MRC. This is NIH funded. Collaboration with Drs. John Walker and Andrew Leslie, Cambridge, U.K. Of course, this is old news, but we are in close collaborative studies on the yeast F1- ATPase with Drs. Walker and Leslie. Upcoming Meetings Gordon Research Conference in Bioenergetics, 2021 Yeast Meetings, 2020 and beyond. Argonne APS ______________________________ "Cogito cogito, ergo cogito sum" ______________________________ "Best Title" Award Goes to: Power, Sex, Suicide: Mitochondria and the Meaning of Life by Nick Lane, Oxford University Press, Oxford, U.K., ISBN 0192804812
"Genius is 1% inspiration, 99% perspiration" Thomas Edison
|